The FDA has an “Ultra Vires” Problem.
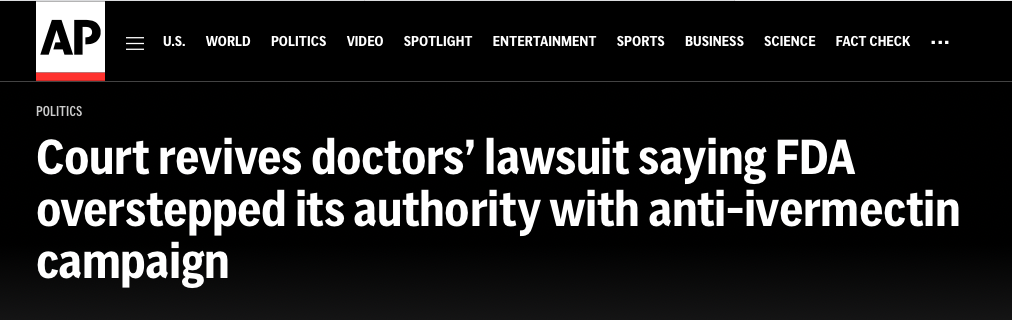
An appeals court found that the FDA overstepped its authority in a public campaign against treating COVID-19 with the anti-parasite drug ivermectin.
“So let me get this straight,” I said to members of our FLCCC marketing team as we met on a Zoom call one morning in the spring of 2022. “Three doctors—including our Dr. Marik— are suing the FDA? Can they even do that?”
“Well, whether they can or not remains to be seen,” said a member of the team. “But yes, their suit is about to be filed.”
Whoa. This looked to me like the plaintiffs were about to grab a tiger by the tail. Three doctors—Robert Apter, MD, Mary Talley Bowden, MD, and FLCCC co-founder, Paul E. Marik, MD—filed suit against the FDA in the U.S. District Court on June 2, 2022. In the suit, the three alleged that the FDA acted “ultra vires” —outside of its authority—to illegally interfere with the doctors’ ability to practice medicine because of the FDA’s aggressive public media campaign to stop the prescribing of ivermectin for the prevention and treatment of COVID-19.
Ok. So let’s assume that what the doctors alleged was true. (It is…but more about that in a moment.) I had always thought that governments—both federal and state—enjoyed “sovereign immunity”…and therefore they could not be sued. (As an aside, it is interesting to note that the idea of sovereign immunity in U.S. law had its roots back in the mother country. The English had adopted a common law known as ‘maxim rex non potest peccare’, which means “the King can do no wrong.” The law was proclaimed by King Charles I in 1649. Hm-m-m. Last time I checked, we have yet to elect a sovereign here in the colonies. But I digress…)
Anyway, if the FDA was technically immune from becoming a defendant in a lawsuit, how did this suit get filed — and ultimately make its way into a court? Well, it seems there have been exceptions that do allow for prosecution of a government agency. Perhaps being “ultra vires” would be one of them, because in the end, what the FDA did was illegal.
This is how the FDA came to have a serious “Ultra vires” problem.
The FDA believed it could issue what is said was merely a “consumer alert” (yeah, right) in its campaign against ivermectin —saying that it should not be used for COVID-19. But it did not first engage in the deliberative process that was legally required. There is no statute on the books that authorizes the FDA Office of External Affairs permission to bypass administrative requirements and put out public proclamations about whether a disease can be treated with an off-label medication.
Also, the Court previously issued a binding decision setting clear boundaries for the FDA’s authority in this area: “The FDA does not restrict physicians from prescribing an otherwise FDA-approved drug for an off-label use.” (Uh…but they did.)

ABOVE PHOTO: Despite its protestations in court, the FDA had to know what it was doing when it tweeted this. Why else would they mount such a strong campaign against the drug? The FDA tried to cast all ivermectin as horse medication with this tweet — and then they published directives about human ivermectin, trying to conflate the two. Little wonder then that media across the country (and the globe) began calling ivermectin “horse paste” or “horse de-wormer”. (I might point out that in their postings, they neglected to mention that human grade ivermectin saved millions across the globe during the pandemic. Oh well. Probably just an oversight.)
So here was the FDA, placing itself in the nation’s examination rooms, publicly playing doctor, and telling physicians and patients what already-approved medications should be used and for what purpose. Another instance of acting in excess of its authority — “ultra vires”.
👎🏼 The FDA does not have jurisdiction over the practice of medicine and acted in excess of its authority in explicit violation of its own governing statute. “Ultra vires.”
👎🏼 The FDA’s public campaign against ivermectin convinced organizations such as the Federation of State Medical Boards (FSMB), the National Association of Boards of Pharmacy (NABP), the American Medical Association (AMA), the American Public Health Association (APhA) and the American Society of Health System Pharmacists (ASHP) to join the FDA’s efforts to restrict physician prescribing.
👎🏼 One of the real harms of the FDA Campaign against ivermectin was making the public believe that it indeed had conducted fact-finding research to determine if the use of ivermectin in COVID-19 patients was indeed safe or effective. It had not.
👎🏼 Despite all of this, last November, the U.S. District Court dismissed the lawsuit, saying that the FDA did indeed have “sovereign immunity.” It was the court’s opinion that the FDA enjoyed absolute protection from any wrongdoing or harm in directing the public not to use ivermectin.
But then…
👍🏼 The plaintiffs filed a swift appeal against the dismissal. In February of 2023, Alan Dumoff, an attorney for the FLCCC, wrote an amicus brief delineating the legal arguments about why the case must be returned to the U.S. District Court for trial. (An amicus brief can be filed in an appeals court to provide additional relevant information in a case. Mr. Dumoff was not representing Dr. Marik or the other plaintiffs in the case. He was solely an ‘amicus curiae’, or “friend of the court,” as a third party with expertise in the case.)
I believe that it was Dumoff’s amicus brief—with its brilliantly articulated arguments— that largely contributed to the three-judge panel in the appellate court to reverse the dismissal on Friday—and remand the case back to district court. The higher court said this of its decision to reverse and remand: “The FDA is not a physician. It has authority to inform, announce, and apprise—but not to endorse, denounce, or advise.”
The arguments Mr. Dumoff presented were irrefutable, material facts about the FDA’s Campaign against ivermectin, which, said Dumoff, has been recognized and acted upon in every arena of health care for what it is: An FDA position against prescribing ivermectin for COVID-19.
From Alan Dumoff’s amicus brief:
“The decision about whether a drug should be prescribed always remains with the physician. The FDA not only does not have the authority, it does not have the mechanisms necessary to weigh risks and benefits and dictate medical standards.”
“Numerous courts have cited directly to the FDA Campaign as an advisory against physicians prescribing ivermectin for COVID-19. While the Agency argues it expressed no opinion about physician prescribing, in cases seeking in hospital access to ivermectin, the judiciary has widely and universally read the Campaign with that very meaning. Regulators unequivocally understood the FDA’s Campaign to be a finding that physicians should not prescribe ivermectin for COVID-19 and acted on that finding. “
“There has also been an inaccurate narrative maintaining the objectively false statement that there is no evidence to support the use of ivermectin in COVID-19. The oft-repeated drumbeat that “there are no scientific studies that show that ivermectin is safe or effective in the treatment of COVID-19” is contradicted by a substantial body of completed research including peer-reviewed meta-analyses. Presently, there are over 99 trials, with 1,089 scientists studying 137,255 patients in 28 countries—including at least 46 randomized controlled trials—which cumulatively show both safety and significant benefit. These studies are summarized at an extensive repository listed HERE and a meta-analysis found HERE.”
So what happens next?
Attorney Dumoff warns that, “Allowing the District Court ruling to stand would upend FDA regulation and allow the imposition of federal control over state medical and public health decision-making.”
I could not agree more. That is precisely why the “Reverse & Remand” order of the higher court is so critical. This trial (not yet scheduled) will likely determine the extent to which the FDA will sit in the exam room with you every time you visit your medical provider. And it will not sit there quietly. The agency will be telling your provider which medicines he/she can prescribe—or NOT prescribe— for you. (Even if the recommended drugs been FDA approved.) So this is not just about ivermectin. It’s about restoring the protections of the sacred doctor-patient relationship— without third-party, government interference.
When the case returns to federal district court, the plaintiffs, Drs. Robert L. Apter, Mary Talley Bowden, and Paul E. Marik will argue the FDA acted “ultra vires”—outside of its authority —and illegally interfered with their ability to practice medicine by directing the public, including health professionals and patients, not to use ivermectin, a drug that is fully FDA approved for human use.
👎🏼 Dr. Apter has been referred to the Washington Medical Commission and Arizona Medical Board by the Iowa Board of Medicine for disciplinary proceedings for prescribing ivermectin to treat COVID-19, and the referrals expressly include copies of the FDA’s publications directing against that use.
👎🏼 Pharmacists have refused to fill Dr. Bowden’s prescriptions for ivermectin, citing FDA directives not to use the drug for COVID-19. She was derided by Houston Methodist Hospital and forced to resign her privileges there as a result. This has further resulted in both reputational and monetary harm, not to mention the abuse she now endures online.
👎🏼 Dr. Marik was forced to resign from his positions at Eastern Virginia Medical School (“EVMS”) and Sentara Norfolk General Hospital—even after developing EVMS’s COVID-19 treatment protocol—for continuing to promote ivermectin to treat COVID-19 after the FDA’s attempts to stop use of those drugs for that purpose. The timing of these injuries occurred immediately following the FDA’s pressure campaign against ivermectin.
The Broader Impact of the FDA’s Smear Campaign
The FDA’s “ultra vires” campaign against the use of ivermectin has been the fuel for innumerable disciplinary actions against doctors, interference with the doctor-patient relationship, and has had a severe chilling effect on the use of life-saving medication for a deadly disease. The FDA argued in court that it did not intend for its Tweets and postings to have that effect.
The hell it didn’t.
You and I both realize that patients believe that the FDA’s pronouncements are authoritative and they want care that complies with such declarations. And you can bet that the FDA knows it too.
Because of the FDA’s campaign against ivermectin, medical providers felt unable to safely exercise their professional judgment—particularly while watching as their colleagues were being forced to resign privileges and positions, and threatened with or subjected to professional disciplinary proceedings. Even today, the majority of medical providers employed by hospital systems are unable to break ranks due to strict treatment policies adopted by the hospitals — which predictably mirror federal “guidelines” that favor consensus medicine.
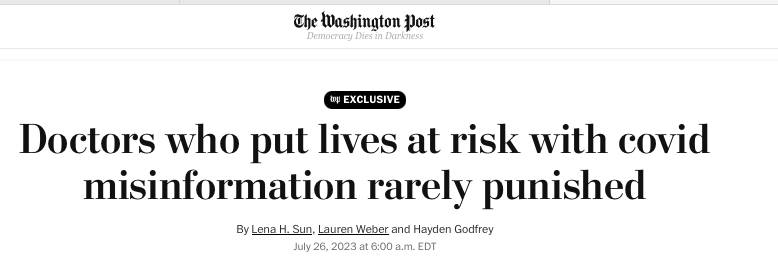
From the Washington Post article calling for increased punishments for doctors and medical providers spreading “misinformation”:
“State medical boards charged with protecting the American public often failed to stop doctors who went against medical consensus and prescribed unapproved treatments for covid or misled patients about vaccines and masks, the Post investigation found.”
And this:
“It is impossible to know how many doctors were spreading misinformation because most states do not monitor or divulge those complaints. But The Post’s requests to the boards yielded at least 480 covid-misinformation-related complaints in the last three years — meaning only a tiny fraction of those led to disciplinary action.”
Oh, the irony of it all. The FDA’s campaign achieved the polar opposite of “protecting the American public.” It is becoming crystal clear that the government was trafficking in its own massive sea of misinformation—to build walls of protection around the COVID vaccine industry and the novel COVID drugs being developed by Big Pharma. (But that’s a deep discussion we’ll have at another time.)
In the end, this is about the patients. It cannot be about anything else.
It is difficult to estimate how many thousands of people lost their lives because the FDA assumed its power was absolute and it could wage an unconscionable war on a drug that saved millions around the world during the pandemic. The lifesaving drug was given to citizens in numerous countries whose public health agencies recognized that ivermectin could —and did—save untold lives.
Tragically, people cannot go into a court of law to get their loved ones back. But here’s what you can do. Watch this case carefully. Write letters to the editor to your local papers about this case. Publish your support of the plaintiffs on social media. Talk about it in your places of worship and community groups…in neighborhood newsletters and with your medical providers.
And as you are able, please consider a gift of any size to support the FLCCC’s ongoing legal fight to bring the perpetrators of this lethal scheme to justice.
Do not back off or back down—even a little.
This is a moment for all of us to speak—loudly—even if doing so frightens you. (Plus, if you don’t let on that you are afraid, then you are so much braver than you think.)
We’ve got this. Onward, friends.





One of the most significant law suits to come out of the pandemic. People globally need a win on this one, as countries around the world tend to follow what the FDA does. Great article.
Powerful & informative article - thank you Joyce for being on the right side of history. The TRUTH can not & will not be censored. Dear Lord, deliver us from evil ...